
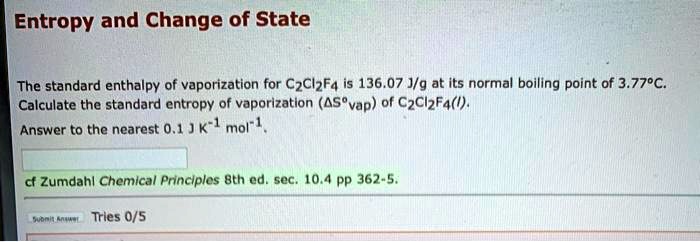
The third law of thermodynamics states that the entropy of any perfectly ordered crystalline substance at absolute zero is zero. Δ Svapm is equal to ratio between the molar enthalpy of vaporisation and the boiling temperature which leads to. To find heat in Calories multiply the mass of the object gaining or losing heat grams by its change in temperature final temp - initial temp by its specific heat for water it is 1 otherwise look up specific heat in book or internet. Delta G systemfree energy change delta H change in enthalpy - Ttemp System represent the change in entropy - q c x m x deltaT The Attempt at a Solution. The heat of vaporization is different for different substances but is a constant for each individual one.
Entropy of vaporization how to#
How to calculate entropy of vaporization.
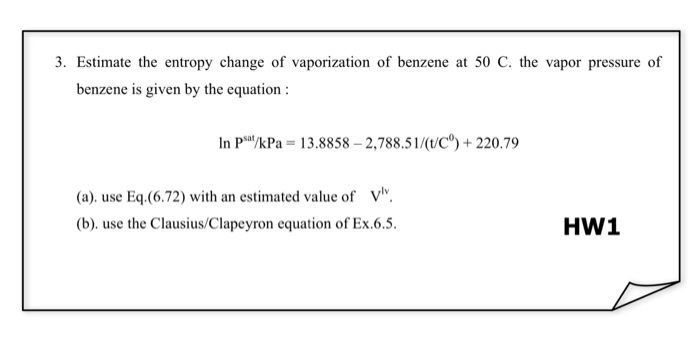
Estimate the entropy a resting person generates in the surroundings in the course of a day at 20C.įor example if the initial and final volume are the same the entropy can be calculated by assuming a reversible isochoric pathway and determining an expression for fracdqT. Homework Equations OK I DONT REALLY KNOW HOW TO a OR EQUATION TO DO IT.


 0 kommentar(er)
0 kommentar(er)
